Triastek’s Latest 3D Printed Drug for Ulcerative Colitis Can Target the Colon Directly
3D printing is increasingly making its way into healthcare, including in pharmaceuticals. Indeed, a few different companies are exploring the use of additive manufacturing to create custom drugs for better treatment of diseases. Now one of the largest, Chinese manufacturer Triastek, has announced a recent success in a first in human (FIH) study for its third 3D printed drug product, T21. It has been proven to deliver the drug directly to the colon for treatment of moderate to severe ulcerative colitis (UC). It is the first time that the colon targeting platform has been verified in human trials according to Triastek.
For those who are not familiar, ulcerative colitis is a chronic inflammatory bowel disease. According to the Mayo Clinic, it causes inflammation and ulcers in the innermost lining of the large intestine, otherwise known as the colon. Though there is no cure, treatment is imperative in avoiding life-threatening complications such as a perforated colon, increased risk of blood clots, severe bleeding, severe dehydration and more.

Triastek is one of the leading manufacturers of 3D printed drugs (photo credits: Triastek)
Indeed, the disease often develops in adulthood and has a prevalence of 156 to 291 cases per 100,000 persons per year. This results in an estimated 600,000 to 900,000 living with the disease in the United States alone. However, as with other gastrointenstinal diseases, though taking medicine orally is the preferred method of drug administration, its effectiveness can vary wildly between patients. This is because the digestive environmental and mucosal transport mechanisms across the gastrointestinal tract can differ substantially. As a result, this can lead to complications such as the medication being released in other parts of the gastrointestinal system and it has often been difficult to achieve precise delivery of drugs to specific locations. This is the exact issue that Triastek is hoping to address with T21, an oral colon-targeted delivery drug which has already received IND clearance from the FDA.
Triastek’s Newest 3D Printed Drug, T21, Can Target the Colon
To create T21, Triastek turned to its self-developed process, named 3D Microstructure for Colon Targeting (3DµS-CT) in order to develop a 3D printed prototype with various combination of multiple release control mechanism. This allowed them to test various effects of time and pH and allowing them to achieve a more precise delivery of the medicine to the colon as they were able to reduce the impact of individual gastrointestinal differences. The technique builds upon Triastek’s Melt Extrusion Deposition (MED) proprietary 3D technology, which has also been used for the creation of other 3D printed drugs from the company. The process is done with a specially-built 3D printer that allows powdered raw materials to be mixed and fused into a movable semi-solid before being extruded and printed layer by layer, resulting in three-dimensional drug tablets.
The FIH-tested T21 tablet consists of multiple layers in order to achieve this precise targeting of the colon. According to Triastek, it is composed of three layers: an enteric layer; a delay layer; and a drug core. To start, the outer layer is the enteric layer which maintains the overall structure of the tablet in the gastric acidic environment. It is pH-dependent for this purpose. Once the tablet has passed through the pylorus to the small intestine, it is rapidly eroded in order to expose the delay layer. This layer is time-dependent and continues to erode at a constant rate in the intestinal tract. This erosion rate can also be adjusted through the thickness of the layer of pre-selected polymer material(s) in order to adapt to each patient. Finally, the tablet should fully erode when entering or within the ascending colon, leaving the drug core. This consists of the active ingredients which should be more effective as they have reached the targeted area for maximum impact.
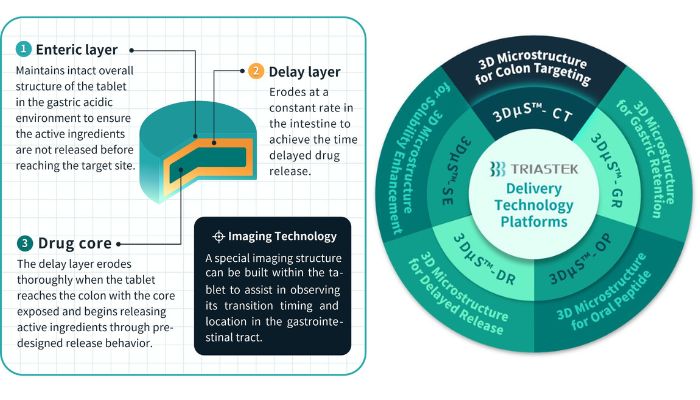
Triastek’s different 3D printing processes (right) as well as the design for T21 (left) (photo credits: Triastek)
Now with this FIH-testing, it has been proven that this tablet is successful in targeting the desired area. Another win for the 3D printed drug market when it comes to treating gastrointestinal diseases. Professor Xiaoling Li, co-founder and chief scientific officer of Triastek, concluded, “The first in human study data with T21 verifies the precise colon delivery capability of the MED process, and this platform is poised to become the novel drug delivery system of choice for colon targeted new product with either local efficacy or systemic absorption. We hope to continue showcasing how Triastek’s 3D printing processes can bring technical solutions to pharmaceutical companies for efficient product development of optimized drug delivery, ultimately leading to the ability to provide patients with more clinically valuable medicines.” You can find out more in Triastek’s press release HERE.
Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly Newsletter here, the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.
*Cover: Chronic inactive ulcerative colitis in a resection specimen (photo credits: CoRus13, CC BY-SA 4.0 via Wikimedia Commons)







