Anatomic Implants Seeks FDA Clearance for World’s First 3D Printed Toe Joint Implant
In partnership with AddUp, Anatomic Implants, a medical device startup in Washington, DC, has announced its intention to submit a 510(k) clearance for the world’s first 3D-printed toe joint replacement. While the use of 3D printed implants within the medical industry is not new, this collaboration could represent substantial progress in the field of orthopedics. Especially given the observed success of this implant.
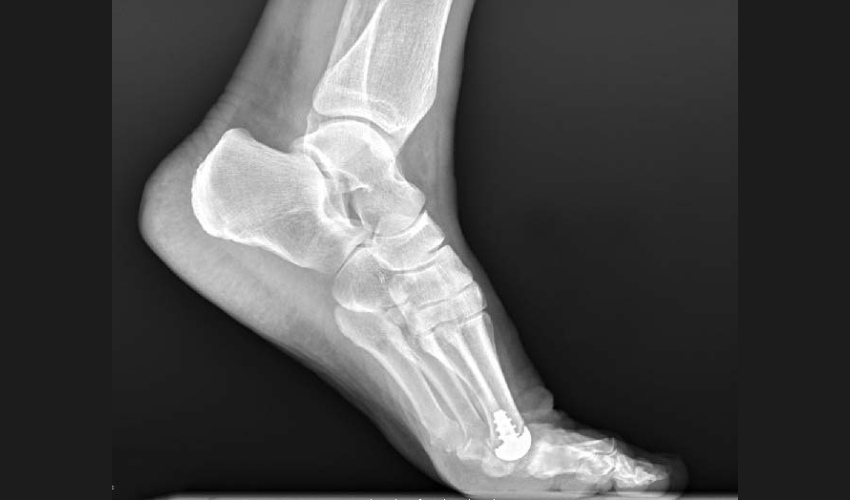
Anatomic Implants has pioneered the development of what they claim is a revolutionary metatarsophalangeal (MTP) joint replacement, strategically utilizing titanium 3D printing technology. This implant is reported to replicate the human anatomy with unparalleled precision, explicitly targeting the 1st MTP toe joint located at the base of the big toe. As the 1st MTP joint is highly susceptible to osteoarthritis, it tends to be the very first joint affected in the foot, quickly leading to significant pain and a substantial loss of balance for those affected.
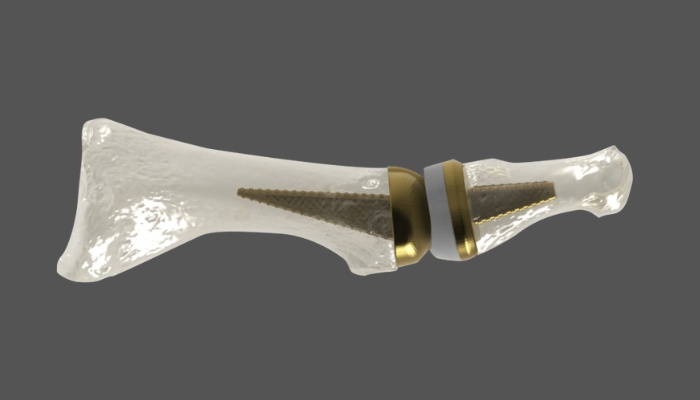
The Anatomic Great Toe Joint implant. (Photo Credits: AddUp)
Despite the annual global market for MTP joint reconstruction exceeding $500 million, there is a severe shortage of viable products to treat osteoarthritis of the joint. The reason behind this? Many are not designed or capable of facilitating bone ingrowth. This often leads to the body rejecting the implant after reconstruction.
Because of the lack of options offered, the market is recognized as a critical, yet hugely underserved domain. This collaboration between Anatomic Implants and AddUp aims to address the unique challenges associated with MTP joint issues. Ultimately, they hope to offer a promising solution for individuals facing such conditions.
Anatomic Implants and AddUp and the First 3D Printed Toe Join
Leveraging additive manufacturing, the companies have incorporated a porous structure into the implant’s design to encourage osseointegration – the connection between living bone and the implant. This crucial feature dramatically enhances the chances of the implant bonding with the bone, minimizing the risk of rejection by the body. This results in significantly improved patient outcomes and well-being after the procedure, showcasing the benefits delivered by state-of-the-art 3D printing technologies.
To produce the implant, Anatomic Implants has turned to AddUp’s FormUp 350 Powder Bed Fusion printer, as it possesses exceptional capability to create complex and custom-tailored designs, meeting the specific needs of the patient’s anatomy. Speaking on the collaboration, Anatomic Implants President David Nutter commented,
We were excited to partner with AddUp to achieve 510(k) clearance after learning about their proprietary 3D printing technology and seeing how it could benefit the development of the Anatomic Great Toe Joint. We look forward to leveraging the AddUp team and their expertise to validate the world’s first 3D printed toe joint replacement on their FormUp 350.”
The 510(k) clearance must be approved before the implant can hit the market. This process includes a thorough assessment of safety and performance data for the product, ensuring it is as safe and effective as any existing market-approved counterparts. Though approval is not expected until the summer of this year at the earliest, the implant will be showcased at the American Academy of Orthopaedic Surgeons (AAOS) Annual Conference in San Francisco from February 13th to 15th.
What do you think about this collaboration between AddUp and Anatomic Implants? Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly Newsletter here, the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.
*Cover Photo Credits: Anika Therapeutics