ZSFab Announces First U.S. Clinical Use of 3D Printed Impants in Spinal Surgeries
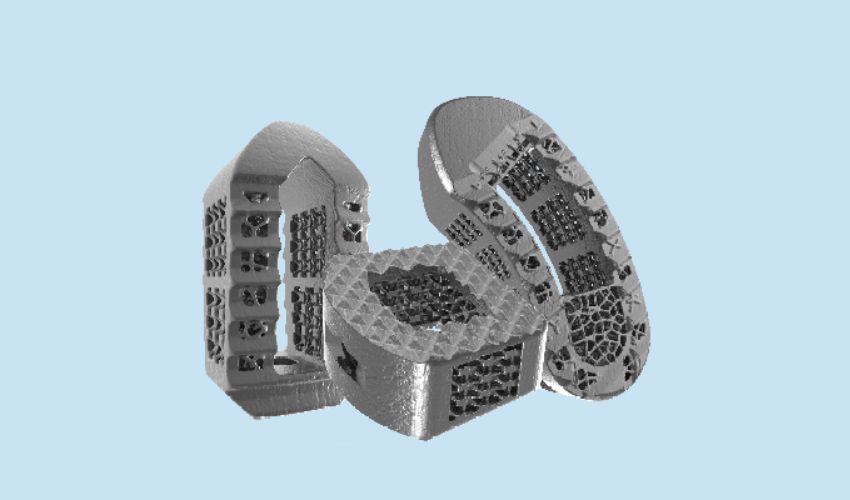
It seems that every week, there is a new innovative use of 3D printing in the healthcare sector. Whether for implants, bioprinted organs, prostheses & orthoses, dentistry, or any of the innumerable applications for additive manufacturing in the wide medical field, it is clear that the technologies have a clear role to play in this sector. Including this latest news! ZSFab has announced the first clinical use of its InterConnect™️ 3D Printed Ti Lumbar Interbody System in the United States.
The Greater Boston-based company is dedicated to the creation of high-performance, patient-matched and customized medical implants, surgical guides and other additively manufactured end-use products. And this is not their first success. Before now, the company has had received FDA 510(k) clearance for its 3D printed lumbar cages and 3D printed cervical interbody systems. With its ZSFab technology, the company employs titanium 3D printing and proprietary algorithms to create orthopedic and spinal devices that are able to promote bone growth while being a better fit to the patient and his or her unique needs.
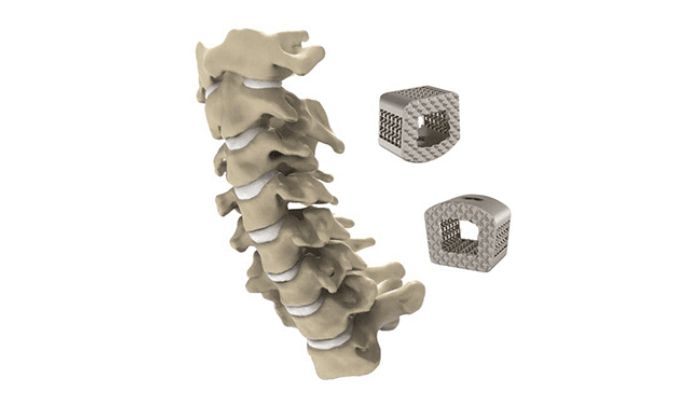
Titanium spinal implant parts made by ZSFab
As Dr. David Ma, Director of Research and Development for ZSFab, describes it, “ZSFab specializes in creating implants that better harmonize with the patient’s biomechanics by addressing issues like load distribution, stress on adjacent segments, and construct stability. To help ensure lasting stability, our optimized designs also help promote beneficial elastic deformation within the lattice structures to induce mechanical stress, activating osteoblasts for new bone growth and achieving a more efficient fusion process.”
For this first clinical utilization, two spine surgeons, Dr. Daniel Harwell and Dr. Michael Thambuswamy of the Oklahoma Spine & Brain Institute, completed three spinal surgeries at Tulsa Spine & Specialty hospital. The implants used were ZSFab’s digitally structured P-TLIF interbody cages, which are designed with state-of-the-art porous titanium. Furthermore, the success of these devices is attribute to a unique combination of triply periodic minimal surface (TPMs) and stochastic lattice structures. Thanks to these factors, the devices are able to enhance osseointegration and promote faster, more robust fusion through the mimicking of the natural architecture of bone according to ZSFab.
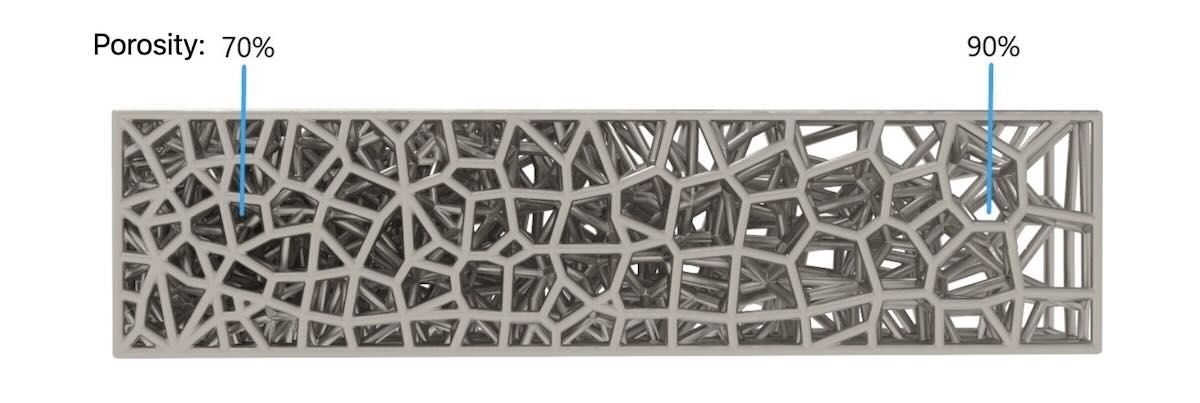
The company is able to customize porosity, pore sizes and modulus according to patients’ bone needs on both periodic and stochastic lattices
Of course, right now it is too early to know the results of these particular surgeries but previous tests all point to promise. Previous HE staining done on ZSFab fusion cages showed clear signs of new bone formed within the porous structures after 12 weeks. In any case, both Dr. Harwell and Dr. Thambuswamy seem very optimistic about the potential results, with Dr. Thmabuswamy especially noting that he is an early user of the companies implant products including cervical and lumbar cages.
Dr. Harwell concludes, “ZSFab’s digital design and optimization platform for implants incorporates clinical needs from the early research phase. I believe this approach enhances innovation efficiency and ultimately ensures excellent long-term patient outcomes.”
What do you think of these 3D printed spinal implants from ZSFab? Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly newsletter here for the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.
*All Photo Credits: ZSFab






