REGEMAT 3D, leader in medical additive manufacturing for nearly 10 years
REGEMAT 3D is a company based in Granada, Spain, specialised in medical additive manufacturing for nearly 10 years. It is undoubtedly one of the pioneering companies in the field of bioprinting in Spain, promoting research and the use of these technologies in the country. This is further proof that we are getting closer to tailor-made medicine every day, not only in the field of prosthetics, implants or orthotics, but also in the bioprinting sector, which will have significant repercussions in the pharmaceutical industry and organ creation. This week we met Jose Manuel Baena, the founder of Breca Health Care and REGEMAT 3D, an expert in the field of bioprinting and personalised medicine. He presents his projects in this sector and talks about the importance of using 3D printing in hospitals.
3DN: Can you present your link with additive manufacturing technologies?

Jose Manuel Baena
I was born in Valencia in October 1983. I studied industrial engineering in Valencia, Spain and then in Braunschweig, Germany and in Oxford, United Kingdom, where I obtained my master’s degree in engineering. I also studied biomedical engineering in Buenos Aires, Argentina, and recently presented my PhD in biomedicine at the University of Granada.
Ten years ago, I started working on a project to manufacture custom implants in a Valencia institute. Then, I decided to found the first Spanish company dedicated to 3D printing of medical devices. I therefore created BRECA Health Care in 2010, dedicated to the production of personalised medical devices. We print 3D titanium implants, with PEEK and other biocompatible materials, prostheses and cutting guides for reconstructions or biomodels. More than 10 years ago, I understood that 3D printing was an obvious need in the health sector, because each patient has a different anatomy, therefore the use of generic prostheses makes no sense.
For custom-made prostheses, the first benefit is the ability to reconstruct the patient’s anatomy with total precision. On the other hand, there is the aesthetic problem. Since we obtain the patient’s geometry by means of a scanner, we can perform “mirror” operations: use their anatomy to create a totally personalised pattern. In addition, we manufacture custom-made guides to perform accurate reconstructions using patient tissues and soon bioprinted materials.
3DN: What were the first steps of REGEMAT 3D in the bioprinting industry?
From BRECA Health Care and, in collaboration with Dr Juan Antonio Marchal, the REGEMAT 3D project, the first development of a Spanish commercial 3D bioprinter, was also launched in 2011. We founded the company in 2015 and currently have users in about 25 countries. We realised that 3D printing made it possible to create three-dimensional cultures and cells from bioinks, which ultimately made it possible to study our cells in an environment very similar to a living organism. On the other hand, it has allowed us to generate tissues and organs in vitro to recreate lesions and to have living models to test drugs. We have therefore begun to invest in the development of these technologies using a highly clinical approach.

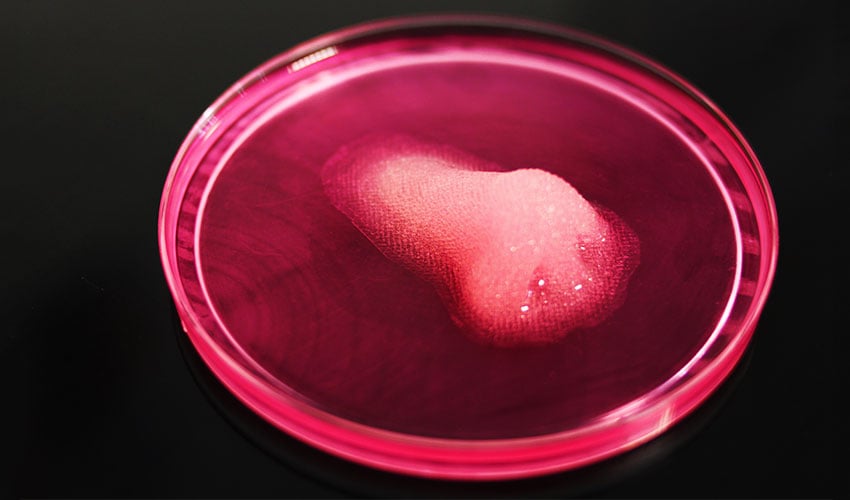
Bioprinted tissues are developing | Credits: REGEMAT 3D
We have positioned ourselves as the first company in the world to adapt bioprinting systems for each specific tissue application and to collaborate with researchers to obtain results that allow the technology to be used in different clinical applications.
With bioprinting, cells behave like a living organism, but it is necessary to properly promote the production of functional tissue. This is why it is necessary to work on the one hand on the biomanufacturing strategy, to adapt the bioprinting system and the bioreactor that matures the fabric so that it is functional: this is what differentiates us from the companies that work in this field.
3DN: What are the projects that REGEMAT 3D is currently developing?
Some of the current applications developed by our users include corneal regeneration, tissue models for drug development, printing of skin, heart tissue, cartilage and bone, and reducing animal testing. Afterwards, we have several research projects with hospitals to manufacture biodegradable meshes with chondrocytes for cartilage regeneration, or to better treat colon cancer by bioprinting tumor cell models. We are also testing some 3D models to regenerate cartilage lesions.
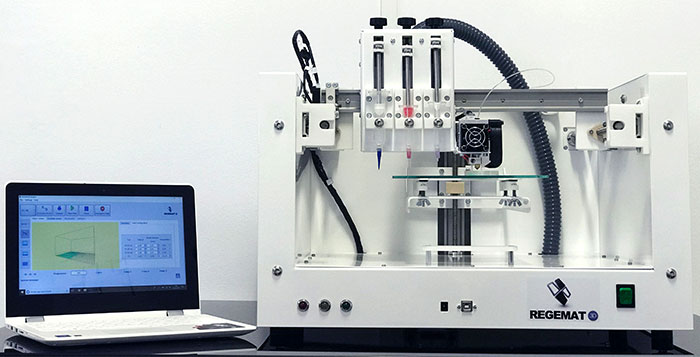
The 3D bioprinter developed by REGEMAT 3D | Credits: REGEMAT 3D
3DN: How do you help hospitals to get started with bioprinting? What role do they have to play in this technology’s development?
Bioprinting is currently only relevant in the field of research, but it will be essential to apply it directly to the clinical phase to provide solutions for patients. Tissue regeneration must be facilitated with the patient’s own cells, which will accelerate the healing of an injury, with less invasive interventions, saving resources and allowing the patient to recover quickly.
We adapt the technology to the application and always from a clinical point of view, because we are the only bioprinting company to implement medical devices and develop 3D technologies with clinical applications in mind.
Through REGEMAT 3D, we help hospitals and research centers take control of the technology, according to each specific application. In addition, we facilitate access to the community of current users to foster contact with researchers and share their experiences.
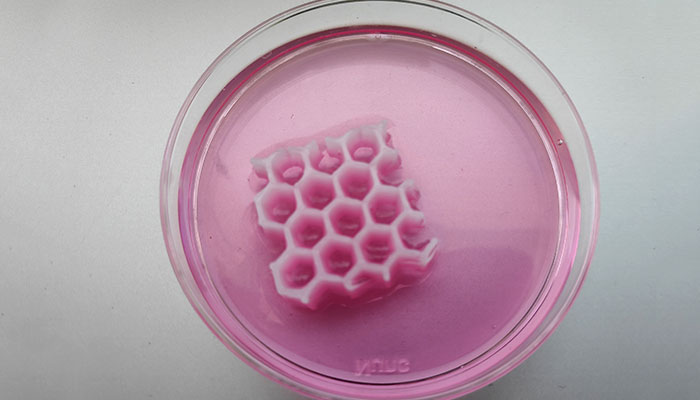
Developing tissues | Credits: REGEMAT 3D
3DN: Where do you see REGEMAT 3D in the coming years?
We see REGEMAT 3D developing complex geometries as complex as our anatomy, with the ability to print meshes to generate the integration of peripheral tissue into medical devices. These technologies are very well accepted in the field of research and, in the coming years, we will obtain new treatments to regenerate wounds and, in the distant future, complex organs to reduce waiting lists for patients requiring transplants. Some independent agencies are forecasting a growth of more than €4,000 million in the bioprinting sector in 2025.
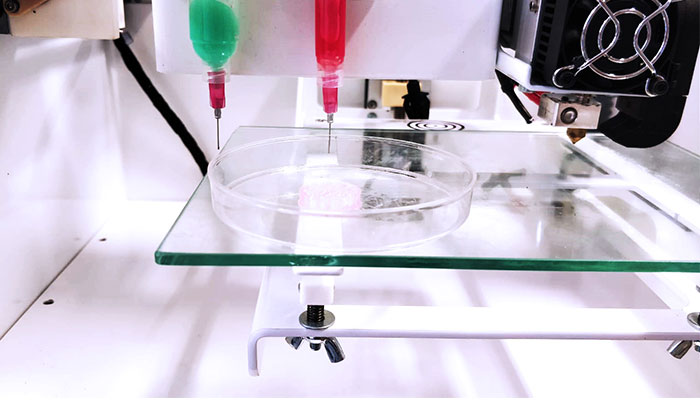
3DN: Any last words for our readers?
New technologies are emerging in Spain’s reference hospitals. 3D printing and bioprinting of fabrics will become part of daily life in hospitals in the next few years. More personalised, specific and successful treatments will help to improve patients’ quality of life and reduce delays and costs for the Spanish health system.
We should point out that currently, even if a functional tissue can be created in the laboratory, integration with the surrounding tissues will not occur instantly: it is necessary to create a customised synthetic medical device to maintain the structure and promote the integration of bioprinted living tissue. Find more information on our official website HERE and in our video below.
What do you think of REGEMAT 3D’s work in the bioprinting sector? Let us know in a comment below, or on our Facebook and Twitter pages! Sign up for our free weekly Newsletter, all the latest news in 3D printing straight to your inbox!






