Renishaw’s 3D printed device delivers successful clinical trials for Parkinson’s
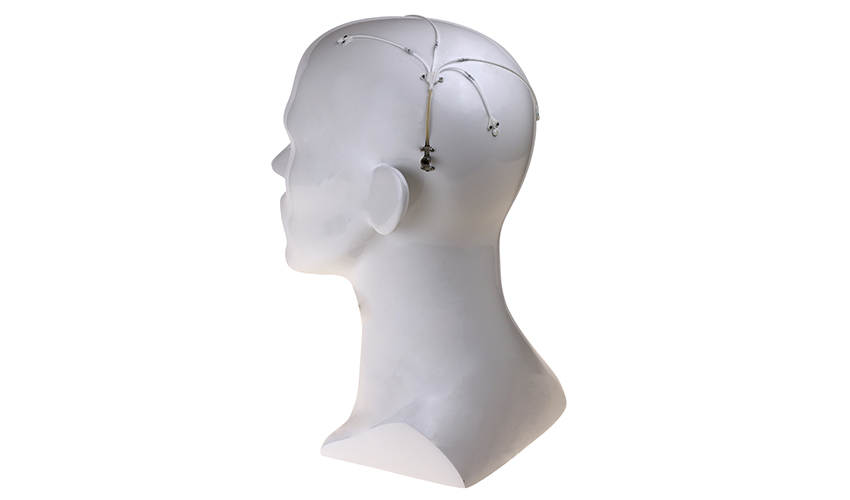
Global engineering company and 3D printer manufacturer, Renishaw, has developed a drug delivery system that could change treatment of neurodegenerative, and other debilitating neurological conditions such as Parkinson’s disease. Parkinson’s is caused by the break-down of dopamine producing neurons in the brain, which leads to involuntary shaking, stiffness of muscles, slowing down of movement and other non-motor symptoms such as memory loss and depression.
Whilst these symptoms can initially be managed with medication, there is currently no treatment available that effectively prevents disease progression, or that treats the motor and non-motor symptoms together. Renishaw’s neuroinfuse™ drug delivery system seeks to provide treatment for Parkinson’s. After the completion of the main part of a clinical trial, the company is positive that their device can play a critical role in treatment for Parkinson’s disease.
Credits: Chris Marshall Mint Motion
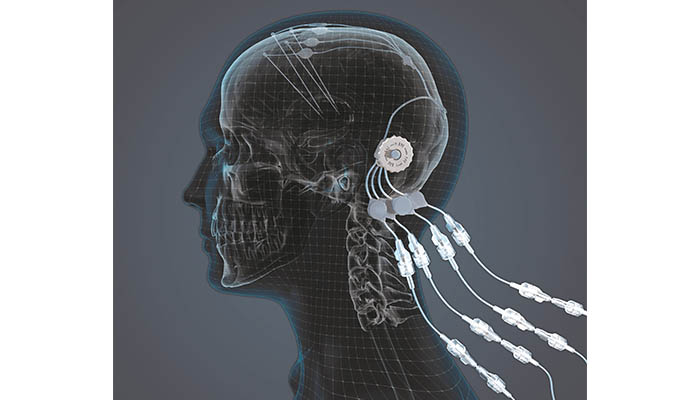
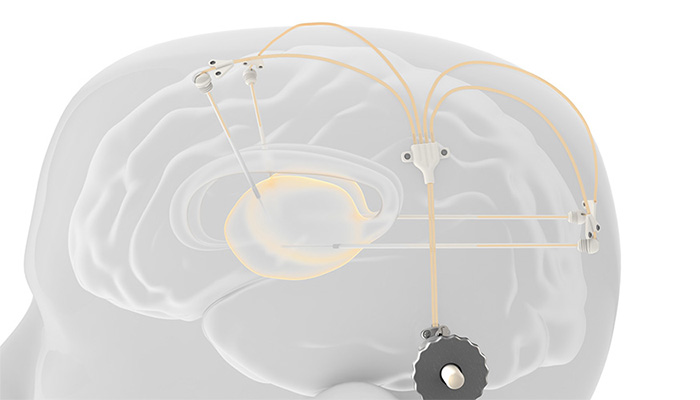
More precisely, it’s intraparenchymal drug delivery offers a practical method of bypassing the blood-brain barrier to deliver drugs to the brain. The device is made up of up to 4 catheters, which can be implanted into target areas within the brain. The catheters are accessed via a 3D printed titanium transcutaneous port implanted behind the patient’s ear, and drug-filled infusion lines are connected using an MRI compatible application set, which repeatedly locates onto the port. Retractable needles extend through a septum in the port to enable therapeutics in the external infusion lines to be infused through the implanted catheters.
This allows patients to receive infusions without requiring the reimplantation of new catheters for each infusion. The repeated regime of this treatment is unique on the market. The initial results of the study with Herantis Pharma plc indicate that the device is performing well. Rupert Jones, Managing Director of Renishaw Medical confirms: “The results of this trial and the performance of Renishaw’s drug delivery system are promising for the many people with Parkinson’s disease.”
The catheters are accessed via a 3D printed titanium transcutaneous port implanted behind the patient’s ear | Credits: Renishaw
During the clinical trials, 17 patients were randomised to receive either 1 dose per month for 6 months of a placebo, or 6 increasing doses of Herantis Pharma plc’s novel drug candidate for the disease. After this 6 month period, patients entered into an additional six-month study where all participants received the drug candidate. As of today, the clinical study has received funding from the EU’s research and innovation program Horizon 2020.
This is not the first time that 3D printing is used for medical implants. Additive manufacturing technologies offer the ability to create precise and custom devices quickly. In this sector, personalization is tremendously beneficial for patients, and many experts predict that tailor-made medical devices will become the norm in the future. You can find more information on Renishaw’s study HERE.
What do you think of these results? Let us know what you think in a comment below or on our Facebook and Twitter pages! Sign up for our free weekly Newsletter, all the latest news in 3D printing straight to your inbox!






