Osseus Announces FDA510 clearance Of New Pisces-SA ALIF System

American medical device company Osseus recently announced the launch and FDA 510(k) clearance of its Pisces-SA standalone Anterior Lumbar Interbody Fusion (ALIF) system. It has been developed to be used with bone screws and alternative fixation bone anchors to enable increased intraoperative flexibility. The ways additive manufacturing is used in the medical sector are fascinating and have managed to help people deal with health issues in ways that haven’t been possible before. The capabilities are vast, reaching from 3D printed prosthetics all the way to ideas of creating 3D printed organs. One area of the medical sector, in which the use of 3D printing technologies is becoming increasingly popular, is the orthopedic industry. 3D printed bone implants offer many benefits, which the new system introduced by Osseus demonstrates perfectly.
The company, based in Dallas, Texas, was founded in 2012 by Eric Hanson and Robert Pace, who had been working in the medical device industry for decades. The main motivation behind the establishment of Osseus Fusion Systems is the development of advanced medical products for spinal-related injuries. By using new, emerging technologies such as additive manufacturing, Osseus steadily aims at the improvement of its products and the advancement of its capabilities, while at the same time maintaining a high level of quality. For that reason, before trying to get the clearance for its Pisces-SA ALIF Interbody System, the company’s researchers conducted a number of tests, which in the end proved that the Pisces-SA anchors provide much better expulsion resistance than others.
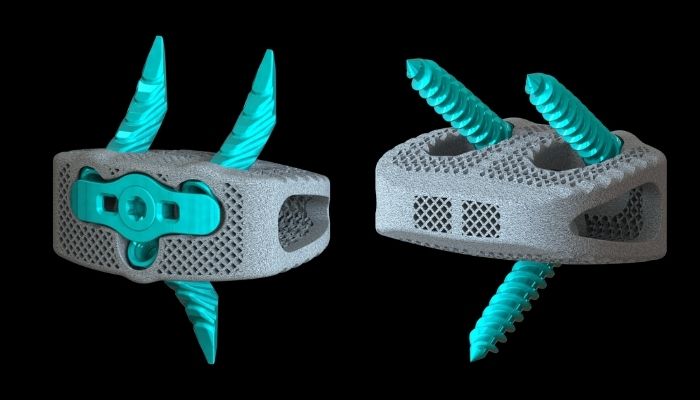
Photo Credits: Osseus
The Innovative and Versatile System
Rob Pace, Co-Founder and CEO of Osseus explains, “Receiving FDA approval for the Pisces-SA is the culmination of relentless work from our R&D department and surgeon design team. We feel this product launch will solidify Osseus as one of the leading innovators in the spine industry. Since our inception, we have pushed the envelope creating minimally invasive products to help simplify and streamline procedures. This product hits that mark, and we are excited to introduce it to the market. This is a special day for our company and for the team of surgeons who have provided extensive input on this groundbreaking product.”
The tests also showed that the anchors perform comparably with traditional screw-based standalone ALIF constructs in stabilizing injured spinal segments. The system, which is said to be the first of its kind to provide this elevated level of expulsion resistance and segmental stabilization, uses an alternative fixation method. The 3D printed spine anchors also integrate a highly porous interbody that supports bone growth, as well as feature streamlined instrumentations, anchor fixation technologies, and a guided locking plate to ensure lock confirmation. The Pisces-SA ALIF system has already received a lot of positive reactions from surgeons and doctors in the medical industry, praising its innovative features and versatility. “I have used screws […], and I’ve used the blade-type constructs, but I’ve never used a device where you could make that choice intraoperatively. I really like that ability to make my decision at the time” Dr. Michael Hisey, an orthopedic surgeon with Texas Back Institute in Plano, says. To find out more about it, visit the company’s website HERE.
What do you think of the Osseus Pisces-SA ALIF system? Let us know in a comment below or on our Linkedin, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly Newsletter here, the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.
*Cover Photo Credits: Osseus






