New Study Examines if EU Regulations Are Stifling Medical 3D Printing

The medical 3D printing market has grown sharply in recent years. This includes in the EU, where the overall healthcare AM market is expected to grow at a CAGR of 15.4% until 2029. Unsurprising when you consider that the European Patent Office found last year that international patent families in 3D printing technologies had grown at an annual rate of 26.4% in the region, with nearly one-fifth of those done between 2001 and 2020 in the healthcare sector. But are there factors that are still slowing its growth?
New research from Dr. Marc Mimler, senior Lecture in Law at The City Law School, seems to suggest so. In the article “Core Legal Challenges for Medical 3D Printing in the EU,” Mimler along with other collaborators from different University hospitals as well as departments of mechanical engineering, sought to examine the EU legislation and case law, falling under Medical Device Regulation (MDR), on issues related to pre-market approval and post-market liability for 3D printed devices. And the results seem to show that gray areas within this are indeed hindering the growth of medical 3D printing, with more work needed to be done to clarify issues.
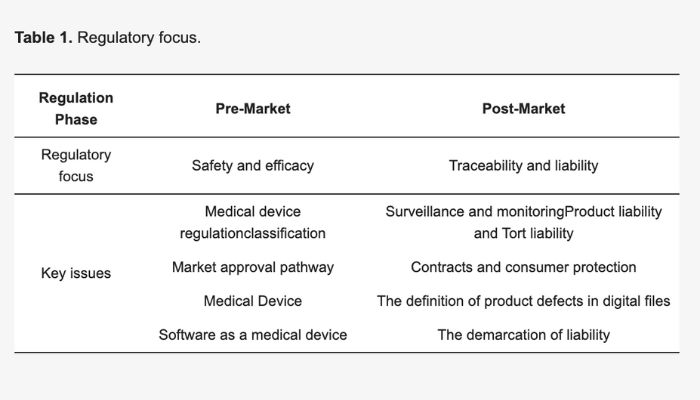
The focus of the study is on pre-market and post-market regulations for 3D printed medical devices (image credits: Mimler et al.)
Are EU Regulations Hurting Medical 3D Printing Growth?
The results, as outlined in a press release from the City School, are divided based on pre- and post-market status. Looking at the pre-market regulations, the researchers focused on the EU’s ‘Custom Device Exemption’ which allows devices made per a written description by an authorized person to bypass requirements like CE marking. And though this would seem a positive for 3D printing, the fact that ‘patient-matched’ devices (which have been made for a specific patient) do not fall under this creates a gray area for 3D printed medical devices. Especially since this distinction is not in place for ‘mass-customized’ devices.
As a result, regulatory compliance is uncertain for deices made using additive manufacturing. This confusion is further compounded by the fact that while industrial manufacturing of 3D printed devices within health institutions and hospitals can fall under the MDR’s Health Institution Exemption, this does not apply to other companies. Making it hard for institutions and smaller companies to navigate the regulatory scene for tailored 3D printed devices.
And that just concerns pre-market approval, post-market regulations also seem to be unclear for 3D printing in the healthcare field. Due to the EU’s more rigorous approach to surveillance and monitoring of medical devices after entering the market, product liability for 3D printed devices is unclear under the MDR. This is due to a blurred boundary between medical negligence and product.
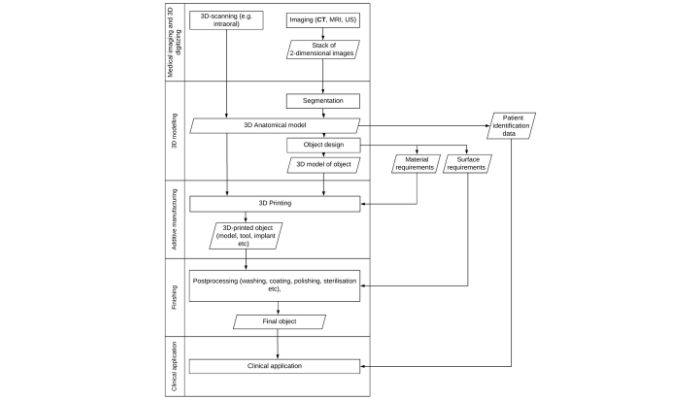
The decentralized nature of the process for 3D printed medical devices (as shown above) can cause confusion and issues for post-market regulations (image credits: Mimler et al.)
This, the authors argue, stifles innovation in the field as parties such as surgeons, third-party manufacturers and in-house manufacturers could be found liable for any defects after the implantation of a 3D printed device in a patient. And the confusion is a direct result of the fact that it is not clear exactly who the manufacturer is due to the decentralized nature of 3D printing. Uncertainty with data protection and intellectual property laws only complicate it further.
All in all, the researchers note that it is important to reexamine what they consider to be conservative and ambiguous requirements under the MDR in terms of 3D printed devices. They argue that currently the system is hampering innovation in the field and delaying care to new patients as actors may not be able to navigate the current regulatory scene for tailored devices and extensive document is also working to slow down time-to-market. You can find out more in the full paper HERE.
What do you think about these findings regarding EU regulations and medical 3D printing? Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly newsletter here for the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.







