3D Printed Human Skin Equivalents Help Identify Potential HSV Treatments
Herpes Simplex Virus (HSV) is estimated to infect 64% of the world’s population, according to the World Health Organization. The virus causes oral and genital herpes, and while it can be asymptomatic, it can also cause painful flare-ups and make those infected susceptible to HIV. Especially for immunocompromised people, the virus can be debilitating. Of course, there are antivirals to treat the disease, the most common being acyclovir—but the treatments are not a cure. Once a person is infected, HSV travels to their nerve cells, where it can start replicating and hide from any antiviral treatments or the immune system. In a few weeks, the immune system can clear up the primary infection, but HSV will remain in those nerve cells it first infected, staying in the body for life. Now, new HSV treatments are being developed thanks to 3D printing.
Current medication is not effective against latent HSV, and it also requires consistent large dosages. Patients can also become resistant to these treatments, which often do not control symptoms adequately. That is why Dr. Jia Zhu, an associate professor in the Fred Hutch Vaccine and Infectious Disease Division who has dedicated her career to studying HSV, is working towards better HSV treatments. In an article for the Fred Hutch Cancer Center, she shared that the key to creating better treatments was understanding why we have our current antiviral treatments in the first place.
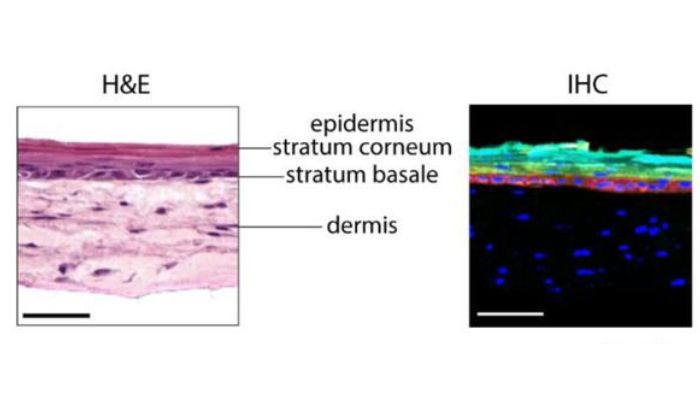
Skin layers (Credit: Zhu Lab)
The type of replicating HSV that can be targeted by antivirals primarily occurs in the basal layer of epithelial cells, which is the innermost layer of the epidermis. This explains why the sores and ulcers associated with herpes often appear in barrier epithelial tissues like the oral mucosa and genital skin. “While this is a complex process facilitated by many different cell types that collectively structure your epidermal tissue,” Dr. Zhu said, “the antivirals we use to treat HSV today were developed using in vitro culture of Vero cells and fibroblasts.”
Vero cells are derived from the kidney of an African green monkey and are one of the more commonly used mammalian continuous cell lines in cell biology research, and fibroblasts are the most common cell type represented in connective tissue. The problem is that this current drug development method captures neither in vivo relevance nor tissue environment. “It’s perhaps not surprising that these antivirals show sub-optimal performance in HSV infections in patients,” Dr. Zhu added.
So, postdoctoral scholars Dr. Ian Hayman of the Zhu lab and Dr. Tori Ellison of the Ferrer lab at the National Center for Advancing Translational Sciences, who are experts in biofabrication using 3D printing technologies led a study in which they 3D bioprinted “human skin equivalents.” These human skin equivalents recreate human skin architecture in a format amenable to antiviral screening and preclinical testing.
How Were the Human Skin Equivalents Made?
According to the Fred Hutch Cancer Center, the Zhu lab 3D printed the human skin equivalents by depositing fibroblasts into specially-designed culture vessels, adding keratinocytes on top of this layer of fibroblasts, and incubating the cells in different media formulations. The result? Small organoids that closely resemble human skin, with dermal tissue (consisting mainly of fibroblasts) and stratified epidermal tissue layers (consisting mainly of keratinocytes).
This is not the first time skin-like material has been 3D printed. In March of 2024, researchers from Penn State 3D printed the first complete and living system consisting of several layers of skin. Before that, other researchers had successfully 3D printed thin layers of skin. However, the Zhu lab’s skin was specially designed for HSV treatment development.
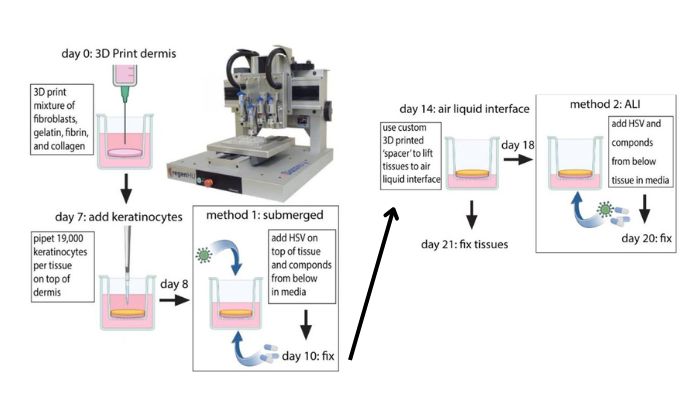
3D printing process (Image Credit: Zhu Lab)
More specifically, the lab developed two types of this organoid system: one to simulate the initial infection of HSV, and another to simulate an HSV flare-up from latent reservoirs. The initial infection was simulated by submerging the model in culture media with HSV. The simulation of latent HSV flaring up was done through an air-liquid interface (ALI) model, where HSV was added to the media side of the tissue, simulating it coming from underneath the epidermis.
Testing Medications with the 3D Printed Skin
Then, in no small feat, the Zhu lab screened 738 compounds for potency against experimental HSV infection. The compounds included novel medications, as well as ones that were FDA-approved. To visualize the effects of the treatments, the team used a recombinant HSV that expressed green fluorescent protein and fibroblasts with a red fluorescent protein. Once the drugs were administered, they could use high-content fluorescent microscopy to track their impact. Drugs that did well eliminating the green HSV signal were on-target, and drugs that reduced the red fibroblast signal were off-target, affecting the host tissue.
The team was surprised by their findings. They discovered almost 20 antiviral compounds that potently suppressed HSV infection with minimal host-cell toxicity. Furthermore, they uncovered significant cell type-specific differences in the potency of novel and existing HSV antivirals, including acyclovir. “We originally used acyclovir as a positive control to screen novel candidate antivirals, but we were shocked to find that acyclovir was at least an order of magnitude less effective in the submerged model (in which HSV mainly infected keratinocytes) than in the ALI model (in which HSV mainly infected fibroblasts),” Hayman said.
Additional testing revealed that the doses of acyclovir required to effectively suppress HSV in keratinocytes were higher than the maximal serum concentrations of acyclovir reported in patients taking the drug. Dr. Zhu added, “Considering that keratinocytes are the major skin cell type in which HSV replicates in patients, the fact that acyclovir isn’t all that potent at suppressing HSV in this cell type may explain why it isn’t always effective in treating HSV flare-ups!”
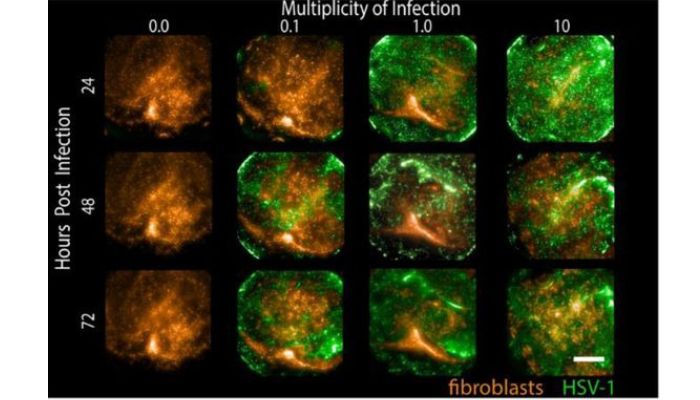
Submerged tissues were infected at various MOI and then imaged at specified times. Fibroblasts express tdTomato (orange) while infected cells express GFP (green) (Image Credit: Zhu Lab)
Medication Development
In the future, the Zhu lab team will continue studying the top antiviral candidates and printing the human skin equivalents, which help them see how antiviral effectiveness is related to specific, patient-derived cell types. Hayman and Zhu concluded, “We’re particularly excited at the prospect of using patient-derived cells to 3D-print the next generation of these skin organoids, because this would allow us to incorporate patient-specific biology into the drug discovery pipeline, and ensure that the drugs we are spending time and money to test are actually showing effectiveness in the cellular environments they will eventually be used in.”
The 3D bioprinted skin platform supported the project by incorporating variable genetic backgrounds early into drug testing. So, they provided a more physiologically relevant approach to identifying potential antivirals for HSV-directed drug development, offering promise and testifying to the advantages of 3D printing. To learn more about the project, find Fred Hutch Center article here.
What do you think of the 3D printed human skin for HSV treatment development? Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! If you are looking for more 3D printing in medical & dental content, check out our dedicated page HERE. Don’t forget to sign up for our free weekly Newsletter here, the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.






