Is 3D Bioprinting the Future of Tailor-Made Medicine?
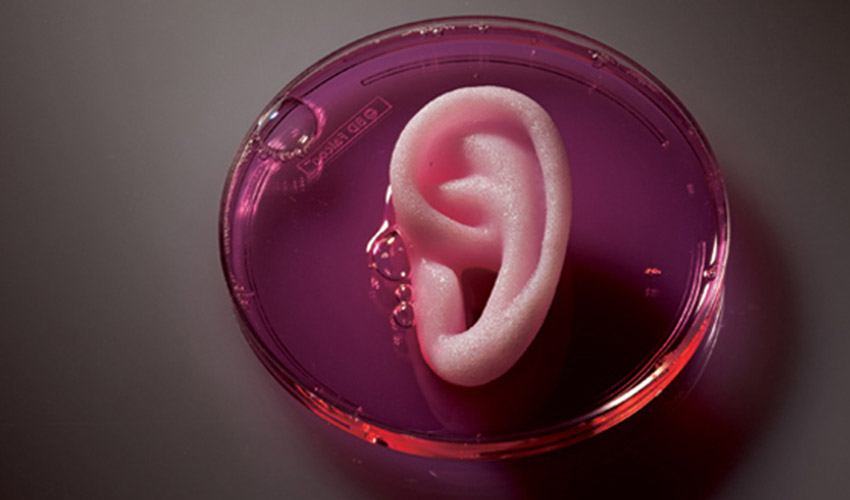
3D bioprinting is revolutionizing the medical field, offering groundbreaking solutions for everything from tissue engineering to personalized treatments. Thanks to 3D printing-like techniques, cells and biomaterials can be combined and deposited layer by layer to create biomedical parts that have the same properties as natural tissues. During this process, various bioinks can be used to create tissue-like structures, which have applications in medical and tissue engineering fields. The varied biomaterials that can be used are combined differently depending on the cells being treated and the final application.
One of the biggest endeavors of this field is to successfully bioprint a fully functional human organ. According to the Organ Procurement and Transplantation Network, around 17 people in the United States die every day waiting for an organ transplant, and the demand for transplants increases every year. 3D bioprinting could be a life-saving solution, but the science isn’t there yet. How could this technology develop? Below, we will explore recurring questions about bioprinting and different printing processes associated with the technology.

(Photo credits: FluidForm)
The Beginnings of 3D Bioprinting
3D bioprinting dates back to 1988 when Dr. Robert J. Klebe of the University of Texas presented his cytoscribing process, a method of micropositioning cells to construct two and three-dimensional synthetic fabrics using a common inkjet printer. In 2002, Professor Anthony Atala of Wake Forest University created the first organ using bioprinting: a small-scale kidney.
To foster bioprinting, Organovo – the first bioprinting commercial laboratory – was opened in 2010 in San Diego, California. The lab quickly began working with Invetech developers to create one of the first bioprinters on the market, the NovoGen MMX. Organovo continues to work on developing bone tissue breakthroughs, even creating tissue for liver transplants.

Professor Anthony Atala
A breakthrough marked the field in 2019 when a team of researchers at Tel-Aviv University (TAU) 3D printed a heart using human cells. This heart perfectly matched the immunological, cellular and anatomical properties of a human patient. Although it was the size of a rabbit heart, its complexity was a first: “In the past, they have been able to 3D print the structure of a heart, but not with cells and blood vessels. Our results demonstrate the potential of our approach for making customized tissues and organ replacement in the future,” explained Prof. Tal Dvir, who led the study. Following the breakthrough marked by the team of TAU researchers, BIOLIFE4D was also able to bioprint a miniature human heart, positioning itself as the first company in the United States to succeed in the feat.
In 2022, a group of researchers at Boston University developed a 3D bioprinted “Miniaturized Precision-Enabled Unidirectional Microfluidic Pump”, also known as miniPUMP. The amazing feature of the miniPUMP is that it can beat on its own, just like a human heart, thanks to its living tissue. This is a significant step in advancing research into the functioning of the heart and certain heart diseases. In addition, other successful small-scale organ reproduction projects have followed in recent years, hinting that research continues in this direction.
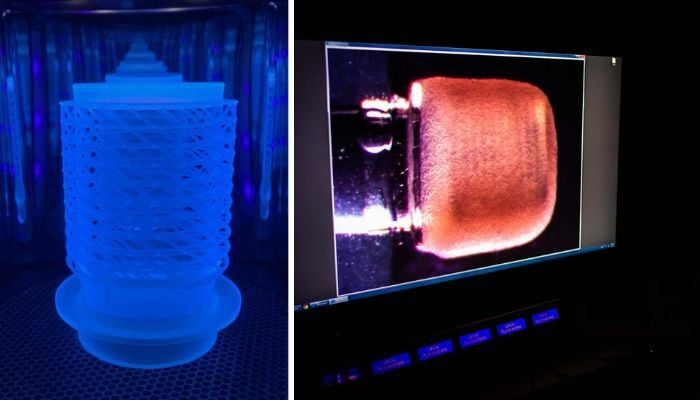
The miniPUMP was designed by researchers at Boston University (photo credits: Jackie Ricciardi)
Of course, for such an ambitious technology there is no shortage of challenges. One of the main limitations is the high cost that often hinders its development, as well as ethical issues. However, today there are new techniques, more or less expensive, that are beginning to take hold.
3D Bioprinting: Process and Techniques
3D Bioprinting can have numerous uses in medicine and medical research. In general, the process underlying each application is collecting, processing, and transforming data to create a design. For the development of functional tissues and implants with human-like morphological and architectural characteristics, it is often necessary to start from the patient’s anatomy.
In simplified terms, the process involves:
- the acquisition of images (CT; MRI, etc.) and patient tissues and cells;
- the reconstruction of damaged tissues or parts to be reproduced using bioengineering software and techniques;
- the selection of the most suitable biomaterial;
- the insertion of cells into the biomaterial and the creation of the printing material;
- and the selection of the most suitable technology for the final application.
Regarding specific technologies, there are three that are generally approved by the international community: Inkjet Bioprinting; Extrusion Bioprinting; and Laser-based Bioprinting. In addition to these, there are other more experimental techniques, some of which we will list at the end.
Inkjet 3D Bioprinting
Inkjet 3D Bioprinting is one of the best-known 3D bioprinting technologies and it is very similar to traditional Inkjet printing. Remarkably, it is possible to modify a classic printer and turn it into a 3D bioprinter with bioinks. Inkjet Bioprinting involves droplets of bioink (also known as biomaterial or biotin) to be deposited layer by layer onto a hydrogel substrate or Petri dish. The technology relies on one of two methods to push bioink out of the nozzle: either thermal or piezoelectric.
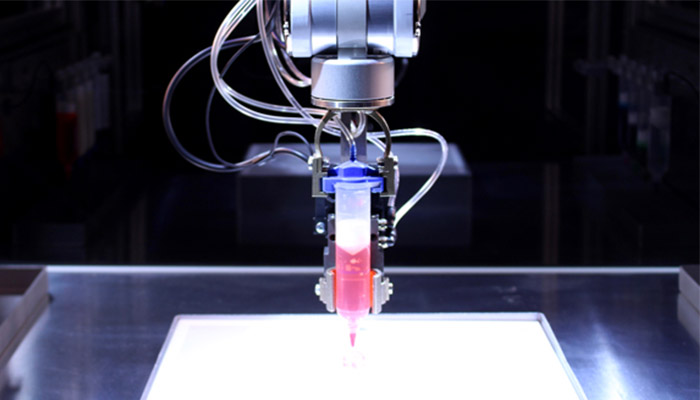
The thermal process employs a heating system to create air bubbles that collapse and provide the pressure to eject the bioink droplets. In the piezoelectric process, a piezoelectric ceramic crystal is present in the nozzle. This material can expand and shrink when an electric current is passed through it. By applying a small electric current, the crystal contracts or dilates, pushing the ink out of the nozzle. A major disadvantage of this technology is that it can damage the cell membrane, leaving one with poor cell-content inks.
Scientists have made great strides in understanding the patterns of molecules, cells and organs with inkjet printing. With these molecules duplicated it is easier to study cancers and potential treatments. Cells that combat breast cancer have also been successfully printed using inkjet bioprinting while retaining their functions, with good prospects for creating living tissue structures or organs.
Organovo relies on inkjet printing to create human models of inflammatory bowel disease. With these models, they aim to understand, invent, and develop the best drug therapies for ulcerative colitis and Crohn’s disease. Their 3D bioprinted tissues mimic key aspects of human biology and disease, and they’ve also done work to reproduce human liver tissues.
Extrusion Bioprinting
Extrusion bioprinting is popular because it is relatively simple to execute and inexpensive. A solution of biomaterials and patient cells is extruded (either through pneumatics, pistons, or air), and layer after layer of the desired model or tissue is printed. One of the advantages of this technology is that it can be done at room temperature. Additionally, cells can be directly incorporated and homogeneously distributed into the print. Some of the most popular bioprinters, such as the Bioplotter and the EnvisionTec, use this technique because it is considered the evolution from inkjet bioprinting.
Allevi 3D Printer (photo credits: Allevi)
Laser-Assisted Bioprinting
Laser-assisted bioprinting uses a laser as an energy source to deposit the biomaterials into a receptor. This technique consists of three parts: a laser source, a tape covered with biological materials and a receptor. The laser beams go through the tape, causing liquid biological materials to evaporate and reach the receptor as droplets. These droplets contain a biopolymer, which retains the adhesion of the cells and helps the cell to begin growing. Compared to other technologies, laser-assisted bioprinting is advantageous because it is a nozzle-free and contactless process. This allows for high-resolution cell printing and droplet control of biotin, but the technology is expensive, and the costs are so prohibitive that they do not allow its wide use and dissemination.
French leader in bioprinting, Poietis, designs laser-assisted bioprinting machines. The company became known for its partnership with L’Oréal, where they tried to recreate a hair follicle to stimulate hair growth. Additionally, Poietis created Poieskin, a model of human skin made entirely from 3D bioprinting. To clarify the laser-assisted bioprinting process, they created a video to show how it works.
Other Techniques and New Developments in 3D Bioprinting
Stereolithography
Stereolithography, or STL, consists of solidifying a photopolymer using ultraviolet light. It has the highest manufacturing accuracy and is suitable for bioprinting because it prints with hydrogels that are sensitive to light. This technology is still under development because of limitations like the lack of biocompatibility and biodegradability of polymers, adverse effects, and the inability to remove supports.

Bioprinting with Acoustic Waves
Developed by Carnegie Mellon University, Pennsylvania State University and MIT, this technology uses ‘acoustic clamps’, a microfluidic device that enables cells or particles to be manipulated using superficial acoustic waves. By using this device, researchers can manipulate where the waves will meet along each of the three axes. At these meeting points, the waves will then form a 3D trapping node that captures individual cells. These cells are collected to create 2D and later 3D patterns. An overall technique that offers high performance in terms of movement accuracy.
Over time this technology has developed, with new applications or techniques debuting fairly quickly. An example of this can be seen at the Northwest University in Illinois with their 3D printed ovary as well as in Sweden, where researchers have successfully 3D printed human cartilage tissue. But while these advancements in the name of medicine have led to exciting talk and speculation about the future, there is another side that has yet to really be touched on, and that is the ethical implications we may face regarding this technology.
The SWIFT Technique
Researchers from Harvard’s Wyss Institute for Biologically Inspired Engineering developed a new bioprinting technique called SWIFT (Sacrificial Writing Into Functional Tissue), as its name suggests this technique enables bioprinting blood vessels on living tissues. In other words, it 3D prints vascular channels into living matrices composed of stem-cell-derived organ building blocks (OBBs).
Rather than trying to 3D print an entire organ’s worth of cells, SWIFT focuses on only printing the vessels necessary to support a living tissue construct that contains large quantities of OBBs, which may ultimately be used therapeutically to repair and replace human organs with lab-grown versions containing patients’ cells. In an experiment, organ-specific tissues were printed with embedded vascular channels using SWIFT and remained alive, while tissues grown without these channels experienced cell death within twelve hours.
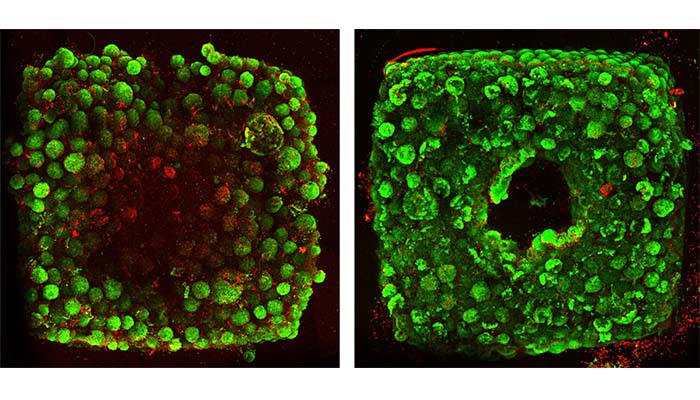
Organ-specific tissues printed with embedded vascular channels using SWIFT remained viable (right), while tissues grown without these channels experienced cell death within 12 hours (left).
The Future of 3D Bioprinting and Personalized Medicine
Doctors are turning to personalized medicine because they are seeking to tailor treatments to their patient’s specific needs. In particular, one of the main applications of 3D bioprinting is the in vitro reproduction of tissues for drug testing and the study of specific diseases. The advantage of this approach is to limit animal testing and increase effectiveness because they are tested on the specific characteristics of each patient.
One example is the work of Carcinotech, which is achieving excellent results by 3D printing tumors derived from patient cells and testing higher-yielding drugs on them. This is a significant step forward in the development of new, more targeted and combination cancer therapies. Creating implants or tissues based on patient cells has also already been successful in clinical cases. One example in this field is the company 3DBio Therapeutics, which makes ear implants with collagen hydrogels and the patient’s cartilage cells.

(Photo credits: 3DBio Therapeutics)
In terms of trends, Grand View Research, an important market research firm based in San Francisco, revealed that it expects the global bioprinting market to reach $4.1 billion by 2026, registering a CAGR of 19.5%. The materials segment is also expected to grow, thanks to other technologies such as AI, scientists can easily determine the right combination of biomaterials, to turn scaffolds into tissues. Bioprinting companies are expected to focus on developing more biomaterials, as well as Bioprinting systems with multiple printheads, to support the use of multiple bioinks in the same print. Bioprinting software is also expected to be upgraded, giving the user more options.
If we look instead at the main present and future concerns, in addition to the associated costs, as anticipated there is also an ethical debate about the consequences of personalized medicine and who will have access to it. Another difficulty from an ethical standpoint is that it is difficult to test the efficacy and safety of these treatments. After analyzing the different techniques used, we know that it would be possible to develop functional organs and tissues that can replace human ones, but it is not yet possible to assess whether or not the patient’s body will accept the new tissue or artificial organ. Not only that, we need to consider the regulations that need to be prepared from a legal standpoint before these advancements are made available to the general public.
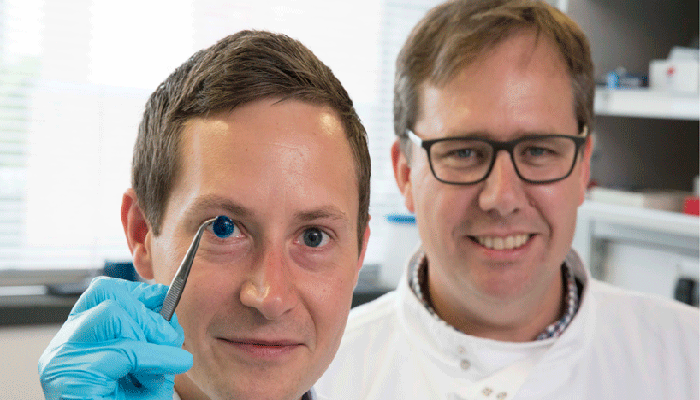
The creators of the first 3D printed cornea (photo credits: Newcastle University)
Moreover, new technologies can always be misused, and 3D bioprinting is no exception. If technologies can create adaptable organs or tissues based on the specific needs of a human being, we need to consider the ethical consequences of this personalized medicine. In particular, we can think about the potential creation of new superhuman capabilities, such as resilient bones or lungs that oxygenate differently.
Do you believe that 3D bioprinting is the future of personalized medicine? Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly newsletter here for the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.






