FDA Approves First 3D Printed Ankle Implant to Treat Rare Bone Disease
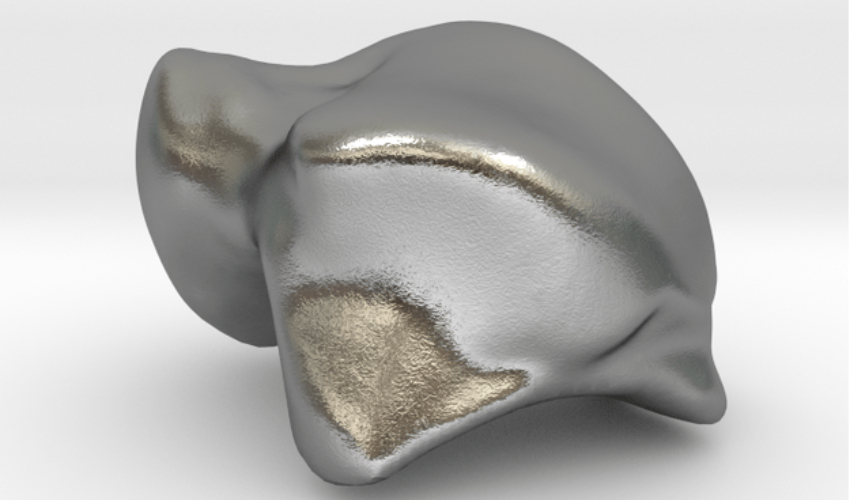
The U.S. Food and Drug Administration (FDA) has just approved the Patient Specific Talus Spacer, the world’s first implant designed to replace the talus. This is particularly useful for sufferers of talus avascular necrosis (AVN). The Patient Specific Talus Spacer is a custom 3D printed ankle implant made from cobalt chromium alloy. Each spacer is modeled from computed tomography (CT) imaging, allowing for it to be fitted to each patient’s specific anatomy.
The talus is the bone in the ankle which connects the knee and the foot. If blood supply to the talus is depleted, the bone tissue surrounding it may die resulting in avascular necrosis (AVN). In most cases AVN results from a dislocation, break, or other sudden injury which inhibits the supply of blood to the bone tissue. This is a serious condition which without treatment can cause the bone to collapse either fully or partially. In order to prevent this, as well as the pain that accompanies the disease, there are two main treatments: the joints in the foot and ankle are fused together, eliminating motion in the joint; or below the knee amputation. This 3D printed implant is a joint sparing alternative, with the potential to significantly improve a patient’s quality of life post-surgery compared to traditional methods.
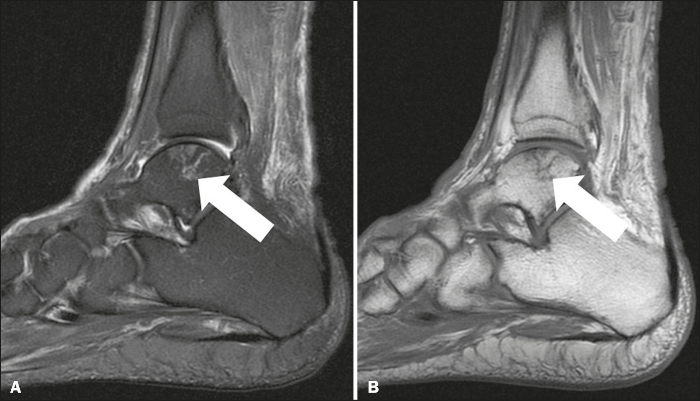
This picture shows avascular necrosis of the talus. (Credit: SciELO)
“Avascular necrosis of the ankle, while a rare condition, is a serious and potentially debilitating one that causes pain and can lead to inhibited motion of the ankle joint, and in some cases, removal of part of the leg,” said Capt. Raquel Peat, Ph.D., M.P.H., USPHS, director of the FDA’s Center for Devices and Radiological Health’s Office of Orthopedic Devices. “Today’s action provides patients with a treatment option that could potentially reduce pain, retain range of motion of their joint and improve quality of life.”
The approval was based off of data acquired from 31 patients and 32 talus replacement surgeries. Before the surgery the average patient was experiencing “moderate to severe” pain. Three years later, not only had the average pain experienced by the patients decrease to “mild”, but patients were also experiencing an improved range of motion in their ankle joints. At this point, only three of 32 cases reported additional surgeries. In fact, the most common reported side effects of the 3D printed ankle implant were pain and scar tissue at the construction site. The FDA have now approved the 3D printed implant as a Humanitarian Use Device (HUD), defined by the FDA as a medical device intended to benefit patients in the treatment or diagnosis of a disease or condition that affects or is manifested in not more than 8,000 individuals in the United States per year. You can read the full press release HERE.
What do you think of the 3D printed ankle implant? Let us know in a comment below or on our Linkedin, Facebook and Twitter pages! Sign up for our free weekly Newsletter here, the latest 3D printing news straight to your inbox!







Good afternoon from Zaragoza Spain. I am doing postdoctoral research on the subject of biomechanical behavior of the subtalar joint to generate a prosthetic prototype of the talus. I would like to know if the article that appears on the 3D Native website: FDA Approves First 3D Printed Ankle Implant to Treat Rare Bone Disease, was taken from a research work published on the matter, since it would be of great use for my review of the state of the art. Thank you very much for your attention and kindness.
The information for this particular article was actually found from a press release from the FDA, which you can access by clicking on the link at the bottom of the article (where it says HERE). That being said, I think you can probably find some more information from the website of the company who make the part, Additive Orthopaedics.