#3DStartup: FemTherapeutics Improves Women’s Health With Personalized Treatment
At the heart of the dynamic healthcare innovation sector, Montreal-based startup FemTherapeutics is positioning itself as a driver of change in women’s health, offering personalized solutions. Specializing in the treatment of pelvic organ prolapse (POP), the company sets itself apart by developing 3D-printed pessaries, perfectly tailored to each patient’s individual needs. We spoke to the company to find out more about its background, methods and future projects. Vincent Castonguay-Siu, head of engineering research and development, told us more about the company’s beginnings, the use of 3D printing technology in this sector and the innovative potential of the startup’s approach.
3DN: Could you introduce yourself and your connection to 3D printing?

Vincent Castonguay-Siu, Head of Engineering R&D at FemTherapeutics.
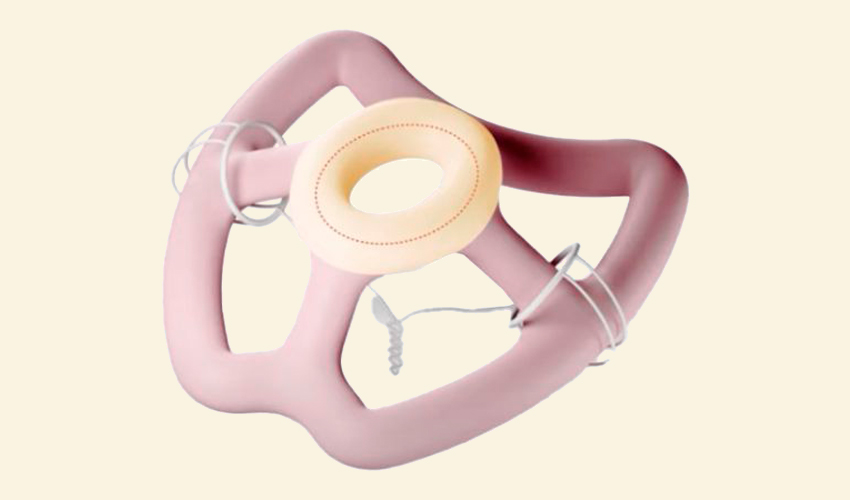
FemTherapeutics is dedicated to revolutionizing women’s health by offering personalized treatment for a condition called pelvic organ prolapse. To this end, we design 3D-printed pessaries specifically tailored to each patient. This customization is based on anatomical and pathological data integrated into our models, enabling us to optimize the shape of the device for each individual.
I’m Vince, head of mechanical engineering research and development at FemTherapeutics. With an M.Sc. in Biomedical Engineering from ETH Zurich, I have seven years’ experience in design and manufacturing, particularly in the field of medical devices. My achievements have been rewarded on several occasions for their design and innovation, both nationally and internationally.
My first contact with 3D printing dates back to my high school and university years. I used the technology to create prototype suspenders for scoliosis, robotic rehabilitation gloves and intervertebral disc implants. However, it was at ETH’s Makerspace, as a volunteer manager, that my passion for creating, teaching and improving came to the fore. Since then, I’ve never stopped creating.
Inara is co-founder and CEO of FemTherapeutics Inc. Before embarking on this adventure, she led Fortune 500 companies through major digital transformations and product launches as a technology consultant at E&Y. Negin Ashouri is co-founder and CTO of FemTherapeutics. She has four years’ experience in robotics and has won several international awards in this field. Finally, Dr Mihnea Gangal is co-founder and medical director of FemTherapeutics. He practices as a urogynecologist at Notre-Dame and St. Mary’s hospitals in Montreal.
3DN: How was FemTherapeutics created, and why did you choose to focus on 3D printing?
FemTherapeutics emerged from McGill’s Surgical Innovation Program, designed to bring together students from multidisciplinary backgrounds and immerse them in the hospital setting to better understand the issues faced by surgeons and patients alike. In the gynecology department, Negin and Inara spent long hours alongside Dr. Gangal in the operating rooms, observing numerous women undergoing surgery to treat a recurring problem: pelvic organ prolapse (POP).
After discussions with their mentor, Dr. Gangal, Negin and Inara identified the shortcomings of existing non-invasive solutions for POP, which were leading to a growing reliance on surgical interventions. It was then that they decided to embark on the development of a dynamic, personalized pessary, aimed at improving patients’ quality of life and comfort.

Negin Ashouri (left), Dr Mihnea Gangal (center), Inara (right).
FemTherapeutics has concluded that 3D printing is the most appropriate technology for the development of personalized medical devices. This technology offers the rare ability to produce customized shapes that meet our stringent quality standards, while remaining economically viable for effective product development.
3DN: What printing processes and materials do you use?
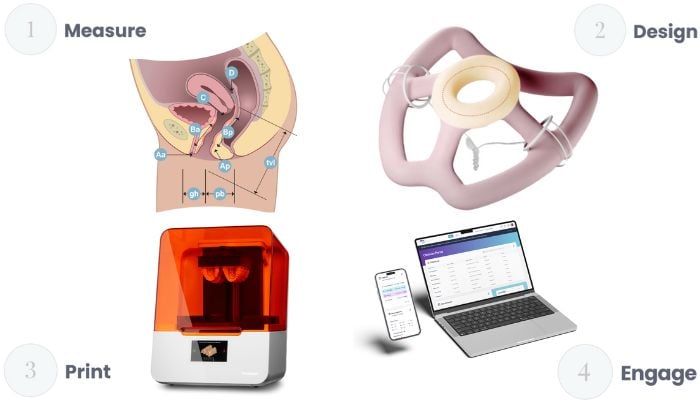
At FemTherapeutics, we generally prefer stereolithography (SLA) 3D printing technology for the development of our pessaries. This preference stems primarily from the ability to produce the complex geometries required for treatment, thus minimizing the need for manual retouching. In addition, the high resolution of this technology, especially with its ability to produce smooth surfaces, facilitates post-processing and reduces the risk of bacterial contamination.
When it comes to materials, we adapt our choice according to the application. For the development of pessaries, we consider the use of medical silicone or similar materials offered by suppliers such as Formlabs, Loctite and Carbon. For functional prototypes, molds and devices, we like to work with technical photopolymer resins such as Elastic 50A, Flex 80A and Tough 2000, as well as filaments such as PLA, PETG and TPEs. Our selection of materials is based on the specific functional requirements of each design, such as stiffness, hardness and surface finish.

3DN: How does the gynecological prosthesis work in the treatment of pelvic organ prolapse (POP) and urinary incontinence?
One in four women suffers from pelvic floor disorders such as incontinence and pelvic organ prolapse (POP). Current treatments include pelvic floor surgery and the use of vaginal pessaries. In the USA and Canada, 20% of women undergo pelvic floor surgery, with a 30% failure rate and increased risk of complications, as evidenced by litigation involving certain types of mesh implants in 2019. Vaginal pessaries are mass-produced intravaginal support devices in geometric shapes and sizes. More than half of pessary wearers abandon their use within the first year due to complications.
FemTherapeutics has created the world’s first adaptable vaginal pessary, offering effective symptomatic relief of pelvic floor disorders. Using the latest advances in iCloud software and artificial intelligence, we have developed a data-driven pelvic health platform. This software allows doctors to visualize each patient’s condition in a 3D environment, then design pessaries tailored to her unique anatomical features. Once the designs are finalized, we use state-of-the-art 3D printing techniques to produce medical silicone directly, an innovation never before used in gynecology.
3DN: What are FemTherapeutics’ future plans?
At FemTherapeutics, we are currently in the freeze phase of the pessary design. Once this has been completed, we will proceed with biocompatibility testing in preparation for a clinical study for regulatory approval in the USA and Canada. We have already frozen the device design and are in the process of finding a manufacturer to obtain samples for regulatory approval and commercial launch, scheduled for the third quarter of 2024.
Our goal at FemTherapeutics is to become the most effective personalized solution for women’s health. Once our first product is finalized, we plan to extend our technology to other areas such as maternal health, oncology and menstruation.
3DN: Any last words for our readers?
As these advances continue, personalized medicine is becoming increasingly widespread. To find out more about FemTherapeutics and our initiatives, please visit our website HERE.

The FemTherapeutics team
What do you think of FemTherapeutics? Let us know in a comment below or on our LinkedIn, Facebook, and Twitter pages! Don’t forget to sign up for our free weekly newsletter here for the latest 3D printing news straight to your inbox! You can also find all our videos on our YouTube channel.
*All Photo Credits: FemTherapeutics







The information you shared is very useful, thank you.