A 3D-Printed System Allows Genes Responsible for Cranial Malformations to be “Silenced”

What if cranial malformations could be treated without surgery, directly at their genetic source? An Italian research team has developed a promising therapeutic strategy to address craniosynostosis, a condition characterized by premature fusion of the skull sutures in infants. The key lies in a “gene silencer” administered via nanoparticles and integrated into a 3D-printed hydrogel that acts locally and is minimally invasive.
In many cases, the most severe forms of craniosynostosis are caused by mutations in the FGFR2 gene, which is responsible for regulating bone growth. When this gene fails, the cranial sutures ossify too early, preventing normal brain development and causing severe deformities of the skull and face, as well as respiratory, auditory, and visual impairments. Currently, these conditions, such as Crouzon syndrome, can only be treated through invasive surgery performed within a few months of life and repeated throughout childhood. The new approach, still in the preclinical phase, aims for something different: correcting the problem at its molecular origin, without major surgical procedures.
Graphic summary of the 3D system for treating cranial malformations (image credits: Lattanzi et al., 2025).
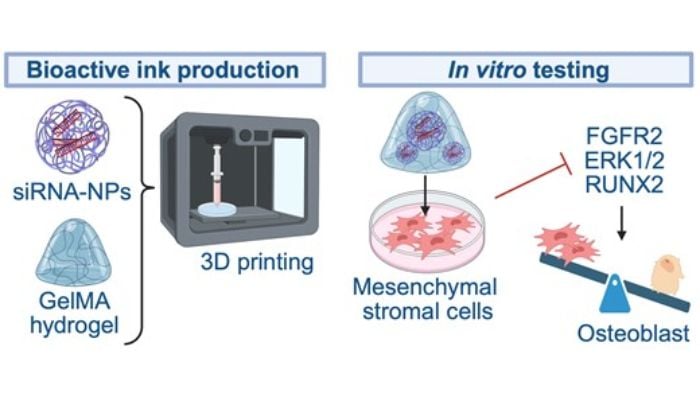
The team coordinated by Professor Wanda Lattanzi, from the Università Cattolica del Sacro Cuore and the Policlinico Gemelli campus in Rome, has been working for years to understand these cellular mechanisms. The most recent result is the development of small interfering molecules, called siRNA, capable of exclusively “silencing” the mutated version of the FGFR2 gene.
Nanoparticles, Hydrogels, and 3D Printing
The question posed by the Italian team was how to deliver this genetic solution to the right cells and maintain its effect over time. This is where nanotechnology and 3D printing come into play. In a second study, published in Regenerative Biomaterials, the team integrated the siRNA into biocompatible PLGA (poly(lactic-co-glycolic acid)) nanoparticles, widely used in medical applications as controlled release systems. These nanoparticles are incorporated into an injectable hydrogel, which can be introduced percutaneously and adapted to the bone defect to be treated. The hydrogel is manufactured using 3D printing, which allows its geometry to be controlled. Once implanted, the system gradually releases the gene silencing, maintaining its efficacy for up to 20 days and reducing the activity of the mutated gene by up to 90%.
Regarding the scientific team’s future plans, they shared that studies in animal models will be necessary, followed by clinical trials of safety and efficacy in humans, a process that usually requires several years of development and regulatory approvals. If everything goes smoothly in the preclinical phase, it is realistic to assume that the first clinical trials could begin within the next five years.
What do you think of the project? Let us know in a comment below or on our LinkedIn or Facebook pages! Plus, don’t forget to sign up for our free weekly Newsletter to get the latest 3D printing news straight to your inbox. You can also find all our videos on our YouTube channel. Interested in more medical and dental 3D printing news? Visit our dedicated page HERE.
*Cover Photo: Università Cattolica del Sacro Cuore







